steel melting process
Basically Steel is made by following methods.
Crucible and high-frequency methods
The crucible process has been superseded by
the high frequency induction furnace in which the heat is
generated in the metal itself by eddy currents induced by a
magnetic field set up by an alternating current, which passes
round water-cooled coils surrounding the crucible. The eddy
currents increase with the square of the frequency, and an input
current which alternates from 500 to 2000 hertz is necessary. As
the frequency increases, the eddy currents tend to travel nearer
and nearer the surface of a charge (i.e. shallow penetration).
The heat developed in the charge depends on the cross-sectional
area which carries current, and large furnaces use frequencies
low enough to get adequate current penetration.
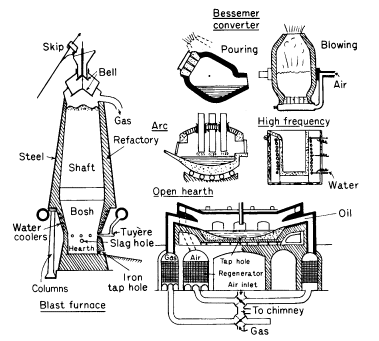
(Figure 1)
Furnaces used for making pig iron and steels. RH side of open
hearth furnace shows use of oil instead of gas
Automatic circulation of the melt in a vertical direction, due
to eddy currents, promotes uniformity of analysis. Contamination
by furnace gases is obviated and charges from 1 to 25 tonnes can
be melted with resultant economy. Consequently, these electric
furnaces are being used to produce high quality steels, such as
steel bars, structure steel, ball bearing, stainless, magnet,
die and tool steels.
Electric arc process
The heat required in this process is generated
by electric arcs struck between carbon electrodes and the metal
bath (Fig. 1). Usually, a charge of graded steel scrap is melted
under an oxidising basic slag to remove the phosphorus. The
impure slag is removed by tilting the furnace. A second limey
slag is used to remove sulphur and to deoxidise the metal in the
furnace. This results in a high degree of purification and high
quality steel can be made, so long as gas absorption due to
excessively high temperatures is avoided. This process is used
extensively for making highly alloyed steel such as stainless,
heat-resisting and high-speed steels.
Oxygen lancing is often used for removing carbon in the presence
of chromium and enables scrap stainless steel to be used. The
nitrogen content of steels made by the Bessemer and electric arc
processes is about 0,01-0,25% compared with about 0,002-0,008%
in open hearth steels.
Acid and basic steels
The remaining methods for making steel do so
by removing impurities from pig iron or a mixture of pig iron
and steel scrap. The impurities removed, however, depend on
whether an acid (siliceous) or basic (limey) slag is used. An
acid slag necessitates the use of an acid furnace lining
(silica); a basic slag, a basic lining of magnesite or dolomite,
with line in the charge. With an acid slag silicon, manganese
and carbon only are removed by oxidation, consequently the raw
material must not contain phosphorus and sulphur in amounts
exceeding those permissible in the finished steel.

(Figure 3). Methods of degassing molten steel
In the basic processes, silicon, manganese, carbon, phosphorus
and sulphur can be removed from the charge, but normally the raw
material contains low silicon and high phosphorus contents. To
remove the phosphorus the bath of metal must be oxidised to a
greater extent than in the corresponding acid process, and the
final quality of the steel depends very largely on the degree of
this oxidation, before deoxidisers-ferro-manganese, ferro-silicon,
aluminium-remove the soluble iron oxide and form other insoluble
oxides, which produce non-metallic inclusions if they are not
removed from the melt:
2Al + 3FeO (soluble) « 3Fe + Al2O3 (solid)
In the acid processes, deoxidation can take place in the
furnaces, leaving a reasonable time for the inclusions to rise
into the slag and so be removed before casting. Whereas in the
basic furnaces, deoxidation is rarely carried out in the
presence of the slag, otherwise phosphorus would return to the
metal. Deoxidation of the metal frequently takes place in the
ladle, leaving only a short time for the deoxidation products to
be removed. For these reasons acid steel is considered better
than basic for certain purposes, such as large forging ingots
and ball bearing steel. The introduction of vacuum degassing
hastened the decline of the acid processes.
Bessemer Method / Metal Refining
Converter
In both the Acid and Basic processes molten
pig iron is refined by blowing air through it in an egg-shaped
vessel, known as a converter, of 15-25 tonnes capacity (Fig. 1).
The oxidation of the impurities raises the charge to a suitable
temperature; which is therefore dependent on the composition of
the raw material for its heat: 2% silicon in the acid and 1,5-2%
phosphorus in the basic process is normally necessary to supply
the heat. The "blowing" of the charge, which causes an intense
flame at the mouth of the converter, takes about 25 minutes and
such a short interval makes exact control of the process a
little difficult.
The Acid Bessemer suffered a decline in favour of the Acid Open
Hearth steel process, mainly due to economic factors which in
turn has been ousted by the basic electric arc furnace coupled
with vacuum degassing.
The Basic Bessemer process is used a great deal on the Continent
for making, from a very suitable pig iron, a cheap class of
steel, e.g. ship plates, structural sections. For making steel
castings a modification known as a Tropenas converter is used,
in which the air impinges on the surface of the metal from side
tuyeres instead of from the bottom. The raw material is usually
melted in a cupola and weighed amounts charged into the
converter.
Open-hearth processes
In the this process, both acid and basic, the
necessary heat for melting and working the charge is supplied by
oil or gas. But the gas and air are preheated by regenerators,
two on each side of the furnace, alternatively heated by the
waste gases. The regenerators are chambers filled with checker
brickwork, brick and space alternating.
The furnaces have a saucer-like hearth, with a capacity which
varies from 600 tonnes for fixed, to 200 tonnes for tilting
furnaces (Fig. 1). The raw materials consist essentially of pig
iron (cold or molten) and scrap, together with lime in the basic
process. To promote the oxidation of the impurities iron ore is
charged into the melt although increasing use is being made of
oxygen lancing. The time for working a charge varies from about
6 to 14 hours, and control is therefore much easier than in the
case of the Bessemer process.
The Basic Open Hearth process was used for the bulk of the
cheaper grades of steel, but there is a growing tendency to
replace the OH furnace by large arc furnaces using a single slag
process especially for melting scrap and coupled with vacuum
degassing in some cases.
The high nitrogen content of Bessemer steel is
a disadvantage for certain cold forming applications and
continental works have, in recent years, developed modified
processes in which oxygen replaces air. In Austria the LID
process (Linz-Donawitz) converts low phosphorus pig iron into
steel by top blowing with an oxygen lance using a basic lined
vessel (Fig. 2b). To avoid excessive heat scrap or ore is added.
High quality steel is produced with low hydrogen and nitrogen
(0,002%). A further modification of the process is to add lime
powder to the oxygen jet (OLP process) when higher phosphorus
pig is used.
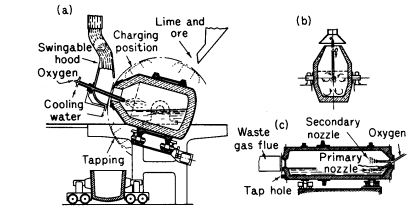
(Figure 2)
|
